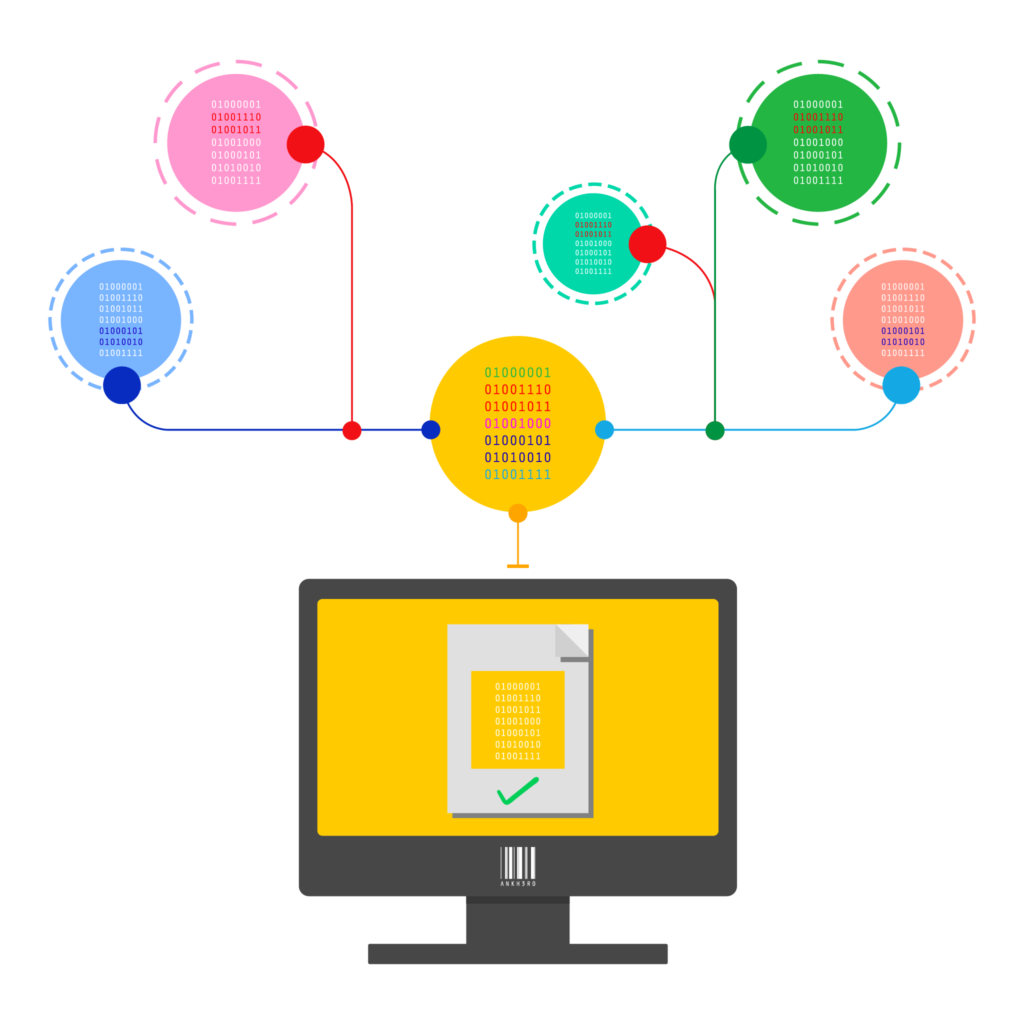
EU ALERT MANAGER
Automated Tracking and Management of EU FMD Alerts
Handling and preventing alerts is easy with the EU Alert Manager.
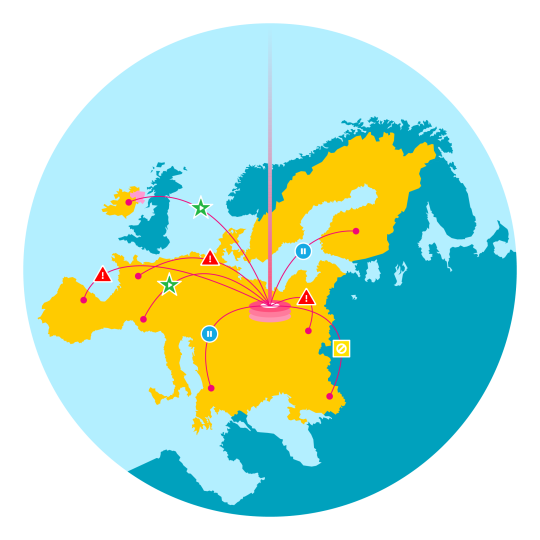
The EU Falsified Medicines Directive (FMD) provides a serialisation and verification framework by which individual items are scanned at the time of dispensing to determine their authenticity.
In situations where these verifications fail, an alert is raised to the dispenser, the National Medicine Verification Organization (NMVO) and the pharmaceutical manufacturer. Alerts may be the result of an unknown product code, an unknown serial number, a mismatch in lot/expiry data and many other causes.
Under the FMD regulation manufacturers have a responsibility to address alerts received, which may include investigating the root cause of the alert, quarantining the product, and maintaining active communication with supply chain trading partners and government agencies.
For manufacturers distributing in many markets throughout Europe, the complexity of alert management is ensuring adherence to the specific requirements defined by each market- including the timeframe in which alerts must be investigated and the necessary authorities who must be kept up-to-date on alert resolution…
EU Alert Manager is the tool to manage any OBP Alert Handling process with the AMS and EU Hub.
EU Alert Manager provides a simple, cost effective solution to help pharma companies manage the entire alert handling process including:
- Automated or manual alert entry
- Management of market specific alert handling requirements - including contact lists for NMVOs and NCAs
- Response tracking and auto reminders ensure alerts are resolved within market-defined timeframes
- Automated summary document generation for trading partner, NMVO and NCA communications
- Full auditing of alert management workflows
- Reporting on alert frequencies, alert types and alert locations
- Web accessible platform for rapid collaboration across functional teams
- Role-based user access ensures data integrity and security
- Direct integration with 3rd party systems such as ERP, regulatory and serialization platforms
- Single-tenant architecture allows for solution customisation
Software
Barcode Generator
Generate Test Barcodes from any compliant EPCIS Message
Master Data Manager
Simple, Cost Effective Master Data and Workflow Management
Services
Supply Chain
Prioritising the most common challenges, ensuring a secure source of supply of medicines.

FMD/Compliance
Covering the EU, UK, US, China and GCC markets, delivering advisory and supervisory services in Regulatory Affairs.

Manufacturing
Delivering Pharmaceutical Manufacturing services, maintaining the continuity of the quality of medicines.
Talk with us
For more information, a free service consultation or software demonstration fill in the form below.